You have submitted and received approval for your IRB application (yay!). Now you have to submit another form to make changes to your original study (amendment), acknowledge a reliance agreement with another institution, request a continuation for your study (renewal/termination form) or terminate your study (renewal/termination form).
All subsequent forms submitted have to be processed through the study page of the initial submission. The initial application established a study page with your study number. Your study number should be connected to every form that is submitted for that particular study thereafter.
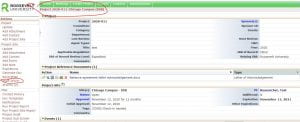
You access your study page by clicking on the hyperlinked study number on your home page. Once you click on your study number, that will take you to a page that looks like this.
Notice that your study number is at the top of the page. When you look on the side column, you will notice the “start x form” in the side toolbar (circled below). Push “start x form” on your study page and that will take you to the next screen.
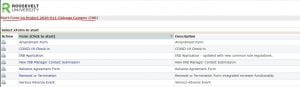
You will see all of the forms you can use in relationship to your study number. From here, you should be able to submit your form.
If for some reason you are not able to access your study page, please contact the IRB Office.
Here is a short video that provides instructions.